1.2.1 Basic Vacuum Physics
Gas molecules are in constant motion within the enclosed vessel (Fig. 11) such as the accelerator guide. Upon striking the vessel surface, they exert a force (F) on the wall of the vessel (A) where these molecular collisions result in a pressure rise. Pressure (P) in a vessel is referring to the impact force (Newton, N) on a unit area (square metre, cm2) caused by the gas molecules hitting the inner walls of the vessel.
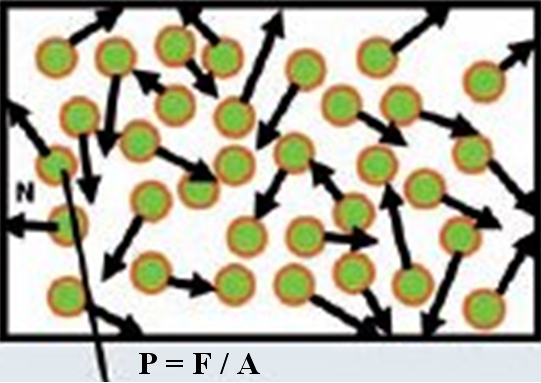
Fig. 11. Constant motion of gas molecules in a closed vessel
Vacuum is an empty space in which the pressure is significantly lower than atmospheric pressure. At atmospheric pressure, surfaces that are constantly bombarded by air and other gas molecules will quickly be contaminated (i.e. low or bad vacuum condition). By removing gas molecules until the pressure is reduced to a suitably low value (i.e. high or good vacuum condition), electrons can travel long distance without collisions with gas molecules in the accelerator guide. The process of removing gas molecules by ion pumps in the accelerator guide is known as evacuation.
To apply vacuum physics in troubleshooting vacuum fault, one should understand the ideal gas law, concept of pressure and vacuum relationship (Fig. 12).
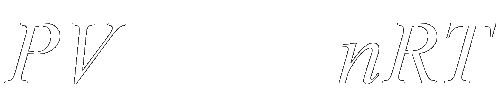 |
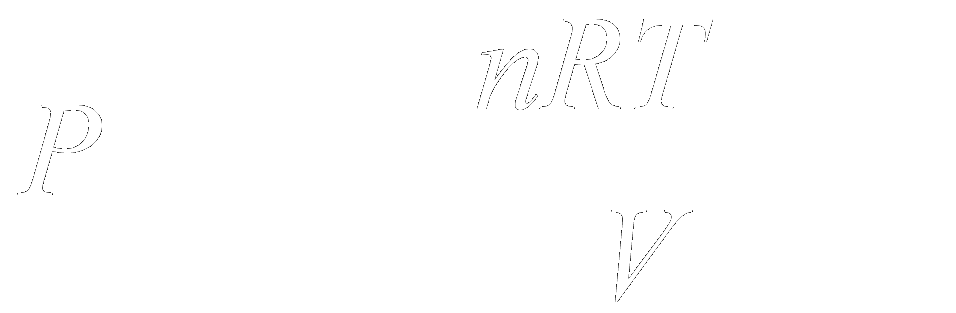 |
The pressure(P) in a vessel can be reduced by increasing the volume (V) of the vessel; decreasing the temperature (T) of the gas in the vessel; or by reducing the number of particles (n) in the vessel. R is the gas constant.
In practical situation, the ideal gas law can be applied to understand why the vacuum activity inside the accelerator guide is maintained.
- The accelerator guide is an enclosed volume under high vacuum condition (i.e. low gas pressure). Any pressure rise is caused by the increase of gas particles inside the guide and temperature of the guide.
- The working ion pumps will get hotter at its stainless steel surface under low vacuum condition. This is a good indication of high gas pressure (i.e. low vacuum) inside the accelerator guide which causes a temperature rise of ion pumps due to more gas molecules hitting its collecting electrodes.
The SI unit for pressure is the Pa (Pascal). The derived unit is the millibar (mbar). Another typical unit of pressure, millimeters of mercury (mmHg), is commonly used to show the atmospheric pressure 760 mm of Hg at sea level. In vacuum work, Torr is in common use as an indication of vacuum condition in linear accelerators. The conversion between different pressure units is 1 Torr = 1mm Hg = 1.333 mbar = 133.3 Pa. A good vacuum in accelerator guide is normally maintained at about 10-7 - 10-8 Torr.
Degree of vacuum which is the measurement of gas pressure can be divided into four pressure ranges:
| Low vacuum | 103 - 100 mbar | 750 - 0.75 Torr |
| Medium vacuum | 100 - 10-3mbar | 0.75 - 7.5-4Torr |
| High vacuum | 10-3 - 10-7mbar | 7.5-3 - 7.5-8Torr |
| Ultra-high vacuum | 10-7 - 10-12mbar | 7.5-8 - 7.5-13Torr |
 |
| Fig. 12. Diagram shows the relationship of gas pressure and vacuum level. |
